LIMS, Laboratory Automation Software and Data Integrity
Today’s technology enables the creation of vast amounts of data. This data must be collected, tracked, managed, retrieved, and used to support compliance. To succeed in this environment, a LIMS is no longer a luxury but a necessity.
LIMS and automation also remove the burden of manual transcription, increasing data quality. Barcoding, instrument integration, sensors, Chromatography Data Systems and enterprise integration all contribute to data integrity by removing user error. As the final product of the laboratory is data, it is critical that the data be accurate and precise. Audit trails are necessary to support compliance with rules regarding electronic records, electronic signatures, 21 CFR Part 11, cGMP, GxP, and additional industry-specific technical and procedural controls.
Data integrity is defined as the maintenance of, and the assurance of, data accuracy and consistency over its entire lifecycle. It is a critical aspect of the design, implementation, and usage of any system that stores, processes, or retrieves data. The purpose of data integrity methods is to ensure data that is recorded exactly as intended data retrieved at a later date is the same as when it was originally recorded.
Physical integrity deals with challenges which are associated with correctly storing and retrieving the data itself, including environmental conditions, power outages, extreme temperatures. Ensuring physical integrity includes methods such as redundant hardware, an uninterruptible power supply, certain types of RAID arrays, and error detecting algorithms known as error-correcting codes. Human-induced data integrity errors are often detected through the use of simpler checks and algorithms.
Logical integrity is concerned with the correctness or rationality of a piece of data, given a particular context, ensuring that the data “makes sense” given its environment. Challenges include software bugs, design flaws, and human errors. Common methods of ensuring logical integrity include constraints, program assertions, and checks.
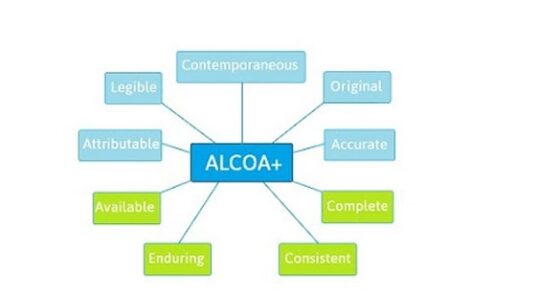
The principles of Data Integrity are defined by the acronym ‘ALCOA’. Data should be Attributable, Legible, Contemporaneous, Original, and Accurate. In addition, ‘ALCOA+’ guidance recommends that data is also Complete, Consistent, Enduring, and Available.
LIMS and Laboratory Automation software play an important role in ensuring Data Integrity with data import, tracking, audit trails, data management, automated instrument integration, automated reporting, importing sensor data (from incubators, fridges and freezers) and traceability. Equipment is, secured in hardened facilities. Expert development teams work on bug fixes, system checks, etc.
Compromised data integrity has serious consequences, including FDA actions–a warning letter, seizure, injunction, or criminal prosecution.
Are you confident in the integrity of your data?
Submit this Form to Subscribe To Our Blog.
By pressing subscribe I agree to receive information about content, events, products, services and promotions from ATL. I agree for ATL to contact me by email to inform me of events, products, services and/or promotions offered. I can withdraw my consent and unsubscribe at any time by emailing info@atlab.com. By submitting my data, I give consent to the collection, processing and use of my personal data in accordance with the ATL Privacy Policy (atlab.com/privacy/).
0 Comments